Polyethylene, non-surgical isolation gowns are easy to put on and take off. Back side is marked and perforated for easy removal. Gap in end of sleeve seal for fingers/hand. Full length, long sleeve style provides full coverage. Polyethylene, non-surgical isolation gowns are easy to put on and take off. Back side is marked and perforated for easy removal. Gap in end of sleeve seal for fingers/hand. Full length, long sleeve style provides full coverage. This Class I device has not been FDA cleared or approved. Not intended for antimicrobial or antivirus protection, infection prevention, or infection reduction, surgical use and is not sterilized. Single use only. Do not use in the presence of any high intensity heat source or flammable gas. Authorized by the FDA under an Emergency Use Authorization for use as PPE in health care settings as they may help protect HCPs and/or patients from the transfer of viruses in low or minimal risk level situations. Apparel Type: Isolation Gown; Material(s): LLDPE; Color(s): Light Blue; Color Family: Blue.
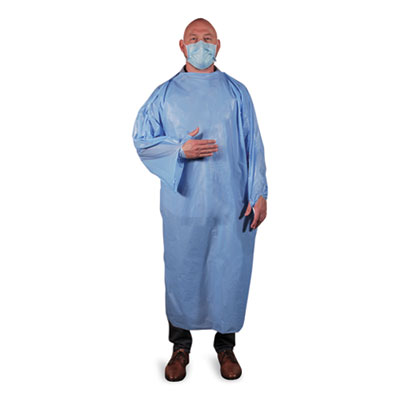
T-Style Isolation Gown, LLDPE, Large, Light Blue, 50/Carton
Login to see price- Easy to put on and take off.
- Gap in end of sleeve seal for fingers/hand.
- Back side is marked and perforated for easy removal.
- Made from polyethylene (Virgin LLDPE).
- Long sleeve, full coverage, one-size.
- Not sterilized, for non-surgical use only.
HERTGOWNLP
HER-TGOWNLP
Additional information
| Brand | HERITAGE |
|---|---|
| Apparel Type | Isolation Gown |
| Global Product Type | Isolation Gowns |
| Material(s) | LLDPE |
| Color(s) | Light Blue |
| Color Family | Blue |
| Size Group | Large |
| Washable | No |
| Suggested Use | Non-Surgical Healthcare |
| Product Biodegradability Indicator | N |
| Product Compostability Indicator | N |
| Compliance Standards | Tested for Compliance with ANSI/AAMI PB70:2012 Minimal Barrier Performance Level 1, Level 2 Requirements |
| Pre-Consumer Recycled Content Percent | 0% |
| Post-Consumer Recycled Content Percent | 0% |
| Total Recycled Content Percent | 0% |
| Disclaimer Statement | This is a Class I device and has not been FDA cleared or approved. |







